Le coin des amatheurs de sciences version 2 
The sections of the site : The site  FORUM
FORUM  The space
The space  The genetics
The genetics  Medicine
Medicine  Physics
Physics  Tintin and science
Tintin and science  Ecology - Nuclear energy
Ecology - Nuclear energy  Mysteries of the history
Mysteries of the history  Humour
Humour  Diverse
Diverse
Le coin des amatheurs de sciences version 2 
The sections of the site : The site  FORUM
FORUM  The space
The space  The genetics
The genetics  Medicine
Medicine  Physics
Physics  Tintin and science
Tintin and science  Ecology - Nuclear energy
Ecology - Nuclear energy  Mysteries of the history
Mysteries of the history  Humour
Humour  Diverse
Diverse
| Physics |
|---|
| The green ray |
| Weightnessless in the elevator |
| The periodic table |
| Physics of the spaghetti |
| Albert Einstein |
| First stage to the invisibility |
If one calls N0 a number initial of radioactive atoms of sample and c the half-life of atom considered (i.e. the time with the end of which half of the atoms are disintegrated), then there are at any moment t :
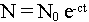
There is also the law :
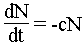
These two relations make it possible to determine the age of object whose one can measure the relationship between two elements (of which one is obtained by disintegration of the other), with the proviso of knowing the initial ratio.
For example, one knows the initial ratio carbon 12/carbone 14 bus this one is constant since the night of times. By measuring it ratio in a rock, one can evaluate its age. One can also use the couple Rubidium 87/Strontium 87, of the couples containing sodium... Moreover, the half-life are of duration extremely different from one core to another (certain species last a few seconds, others like uranium several million years). One can thus choose an isotope adapted to the size-order of the age which one wants to calculate to obtain the most precise possible results: for example, the mummies are often dated with carbon-14 because this one has a half-life of the order of the century.